
They are the electrons involved in chemical bonds with other elements.Įvery element in the first column (group one) has one electron in its outer shell. Those outer electrons are also called valence electrons.
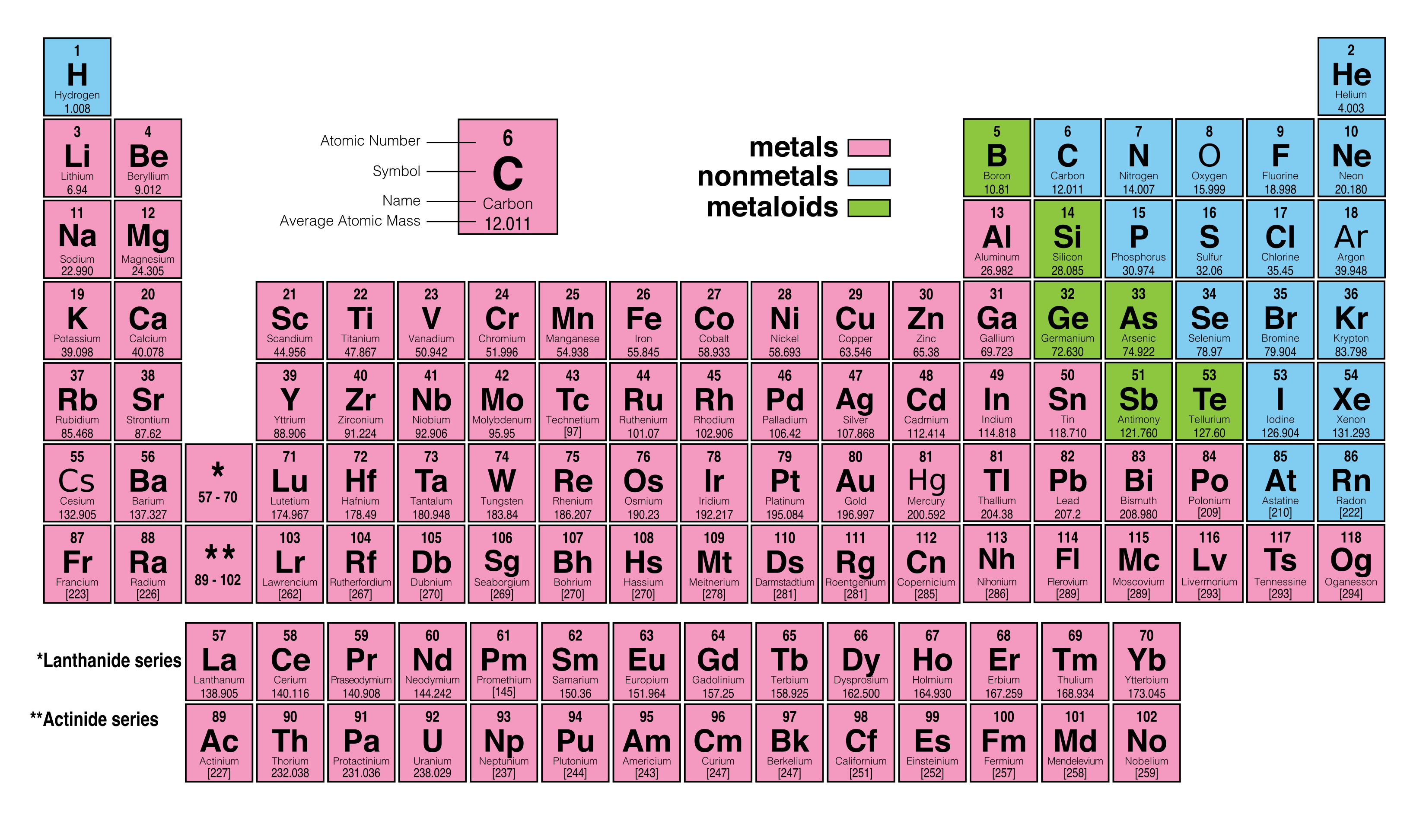
The elements in each group have the same number of electrons in the outer orbital. The periodic table also has a special name for its vertical columns. Now you know about periods going left to right. At this time, there is a maximum of seven electron orbitals. As you move down the table, every row adds an orbital. All of the elements in the second row (the second period) have two orbitals for their electrons. For example, every element in the top row (the first period) has one orbital for its electrons. All of the elements in a period have the same number of atomic orbitals. When you look at the periodic table, each row is called a period (Get it? Like PERIODic table.). Magnesium and sodium (Na) also share qualities because the are in the same period (similar electron configurations).Įven though they skip some squares in between, all of the rows read left to right. For example, magnesium (Mg) and calcium (Mg) are found in column two and share certain similarities while potassium (K) and calcium (Ca) from row four share different characteristics. Each row and column has specific characteristics. As with any grid, the periodic table has rows (left to right) and columns (up and down).
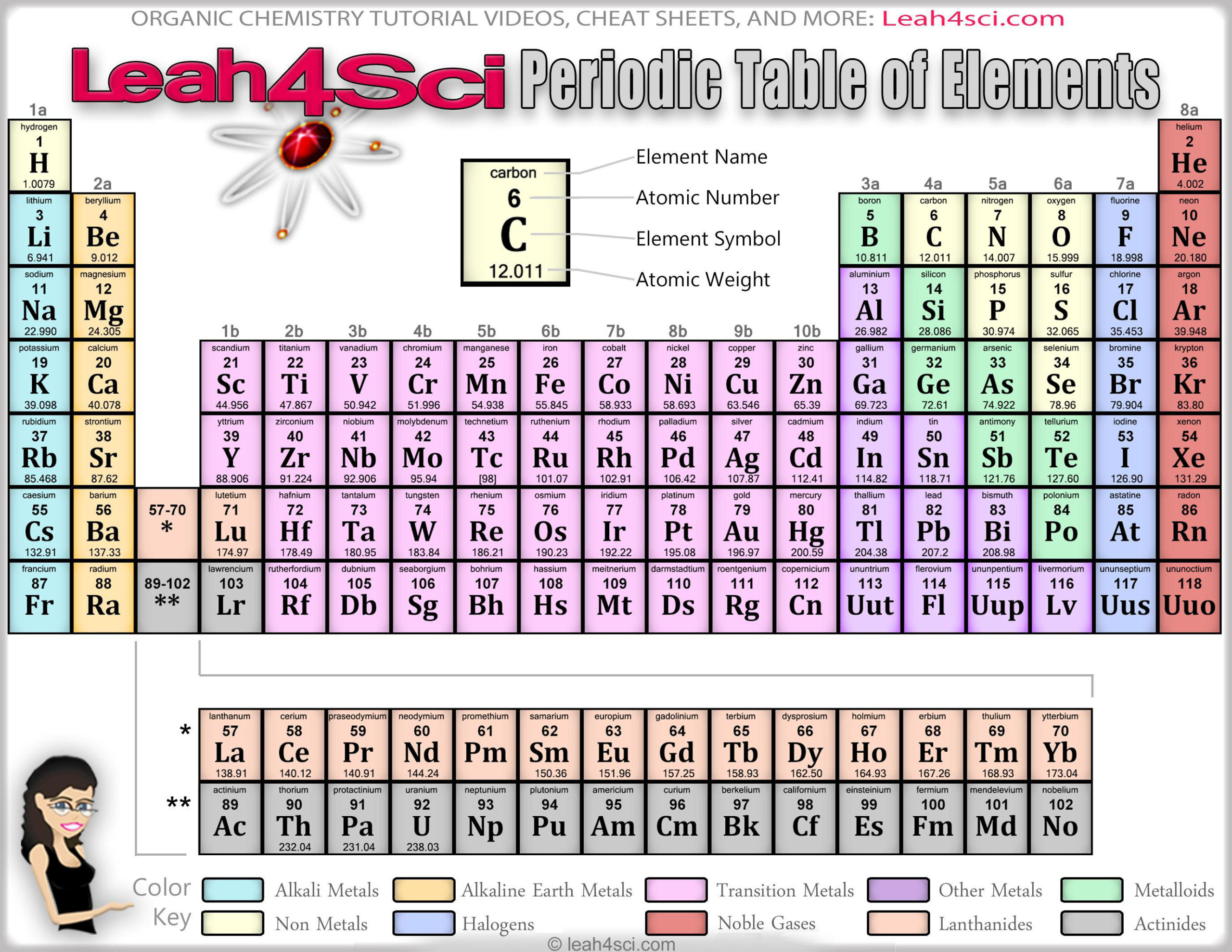
Each element is placed in a specific location because of its atomic structure. The periodic table is organized like a big grid.


 0 kommentar(er)
0 kommentar(er)
